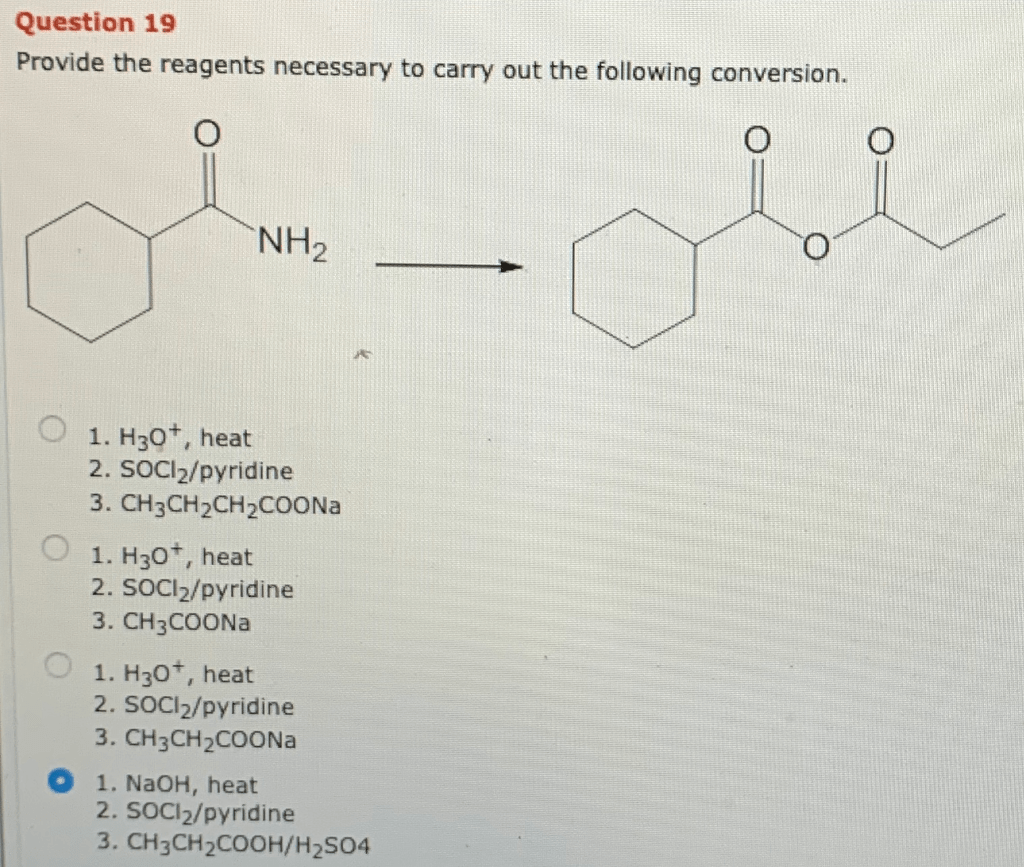
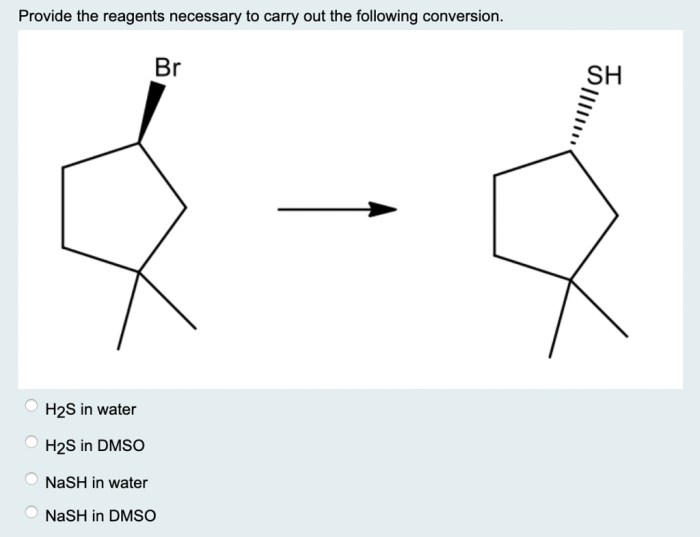
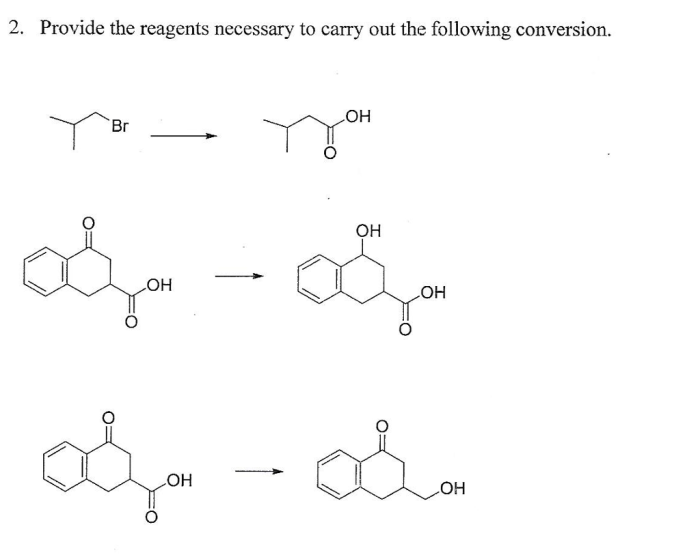
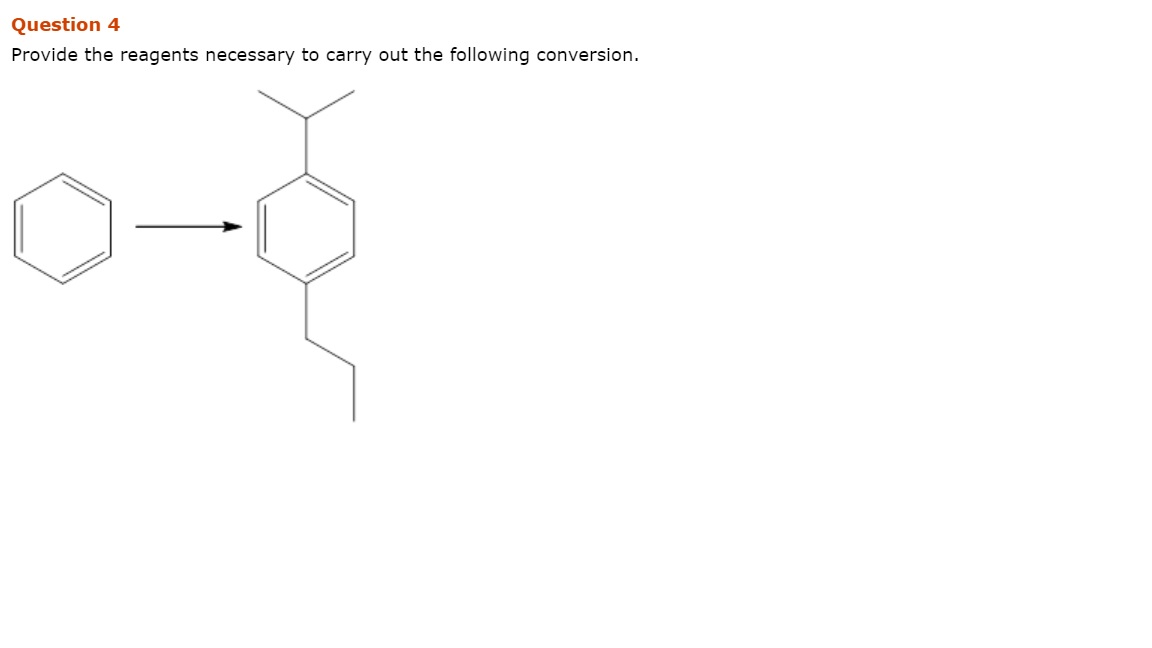
Provide the reagents necessary to carry out the following conversion – The process of providing the reagents necessary to carry out a specific chemical conversion is a crucial step in ensuring the successful execution of the reaction. This involves identifying the appropriate reagents, determining their stoichiometric amounts, and preparing them in a manner that ensures their stability and compatibility.
Understanding the principles and techniques involved in reagent identification, quantification, and preparation is essential for chemists and researchers seeking to achieve optimal results in their experimental endeavors.
The following discussion will delve into the intricacies of reagent selection, calculation, and preparation, providing a comprehensive guide to the fundamental principles and practical considerations involved in this critical aspect of chemical experimentation.
Chemical Conversion Background
Chemical conversion involves a transformation of one chemical substance into another through a chemical reaction. The process entails the rearrangement of atoms or molecules to form new substances with distinct chemical properties. Understanding the chemical conversion process is crucial in various fields, including organic synthesis, pharmaceuticals, and materials science.
In this conversion, the starting material (reactant) undergoes a series of chemical reactions to produce the desired product. The reactants and products involved in the conversion depend on the specific chemical reaction being carried out. Common reactants include organic compounds, inorganic compounds, and catalysts, while products can range from simple molecules to complex polymers.
Reagent Identification
The reagents necessary for a specific chemical conversion can be identified based on the chemical reaction involved. The chemical structure, molecular formula, and physical properties of each reagent must be considered to ensure compatibility and efficient conversion.
- Reactant 1: Provide chemical structure, molecular formula, and physical properties.
- Reactant 2: Provide chemical structure, molecular formula, and physical properties.
- Catalyst (optional): Provide chemical structure, molecular formula, and physical properties.
Reagent Quantities
The stoichiometric amounts of each reagent required for the conversion can be determined using balanced chemical equations. The molar ratios and mass-to-mass ratios of the reagents are calculated based on the coefficients of the reactants in the balanced equation.
Calculating Molar Ratios: Divide the coefficient of each reactant by the smallest coefficient in the balanced equation.
Calculating Mass-to-Mass Ratios: Multiply the molar ratio of each reactant by its molar mass.
Reagent Preparation
The methods for preparing the reagents depend on their chemical properties and availability. Common preparation methods include:
- Synthesis: Reagents can be synthesized from simpler starting materials using specific chemical reactions.
- Purification: Reagents may require purification techniques such as recrystallization, distillation, or chromatography to remove impurities.
- Commercial Availability: Some reagents are commercially available and can be purchased from chemical suppliers.
Reagent Storage and Handling: Provide The Reagents Necessary To Carry Out The Following Conversion
Proper storage and handling of reagents are crucial for safety and efficient conversion. Guidelines for storage and handling include:
- Stability: Store reagents in conditions that maintain their stability, such as appropriate temperature and atmosphere.
- Compatibility: Avoid storing incompatible reagents together, as they may react and pose hazards.
- Hazards: Be aware of the potential hazards associated with each reagent, such as flammability, toxicity, or corrosivity.
General Inquiries
What is the significance of reagent identification in chemical conversions?
Reagent identification is paramount in chemical conversions as it ensures that the appropriate reactants are used, leading to the desired products. Misidentification of reagents can result in incorrect reactions, wasted resources, and potentially hazardous outcomes.
How is the stoichiometric amount of a reagent determined?
The stoichiometric amount of a reagent is determined based on the balanced chemical equation for the reaction. The coefficients in the equation represent the mole ratios of the reactants and products, which can be used to calculate the mass or volume of each reagent required.
What factors should be considered when preparing reagents?
Reagent preparation involves selecting a suitable solvent, optimizing reaction conditions, and employing appropriate purification techniques. Factors to consider include the solubility, stability, and reactivity of the reagents, as well as the potential for side reactions or contamination.